You've likely seen headlines like "Reverse Your Biological Age," and "Turn Back Your Cellular Clock." While much of the anti-aging industry centers around bold promises and expensive interventions, there's fascinating science behind one key concept that keeps appearing in most of these discussions: telomeres.
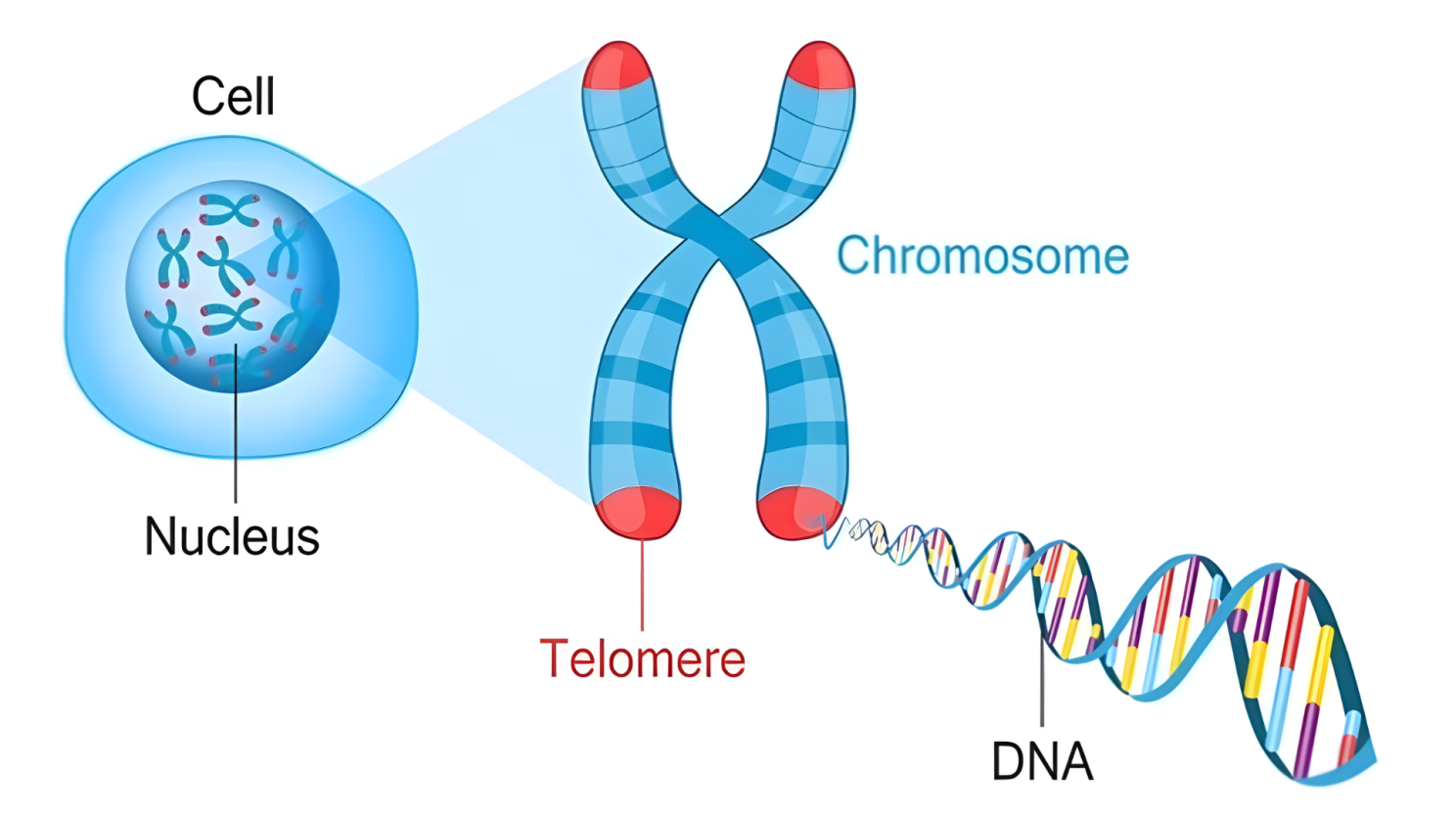
Telomeres can be described as microscopic structures at the tips of your chromosomes that function as cellular aging indicators. Think of them as your cellular aging system that influences how your cells age, how well they can repair themselves, and ultimately how your body maintains itself throughout your lifetime.
Every cell in your body contains 46 chromosomes packed with your genetic information. At the end of each chromosome sits a telomere acting like a protective cap. As you age and your cells divide to replace old or damaged tissue, these telomeres gradually get shorter. When they become too short, your cells can no longer divide effectively, which directly impacts your body's ability to heal and regenerate.
Understanding what telomeres actually do and what legitimately affects them offers valuable insights into cellular health.
Telomeres are specialized DNA-protein structures that cap the ends of chromosomes, much like the plastic tips on shoelaces prevent fraying. They consist of repetitive DNA sequences that don't code for any proteins but serve a crucial protective function. Each time a cell divides, the machinery responsible for copying DNA cannot fully replicate the very ends of chromosomes, causing telomeres to shorten with each division.
This shortening process serves as a cellular counting mechanism. When telomeres become critically short, cells can no longer divide safely and enter a state called senescence, where they stop reproducing but remain metabolically active. While this prevents potentially dangerous genetic errors from spreading, it also limits the body's ability to repair and regenerate tissues.
This is why you might notice slower wound healing as you age, changes in your skin's elasticity and appearance, longer recovery times from injuries, etc. When your cells can't divide effectively to replace damaged tissue, your body's maintenance and repair systems gradually become less efficient.
Research has revealed a clear but complex relationship between telomere length and chronological age. On average, telomeres shorten over time, though this rate varies significantly between individuals and changes throughout the lifespan.
The relationship between telomere shortening and aging isn't linear. The rate of telomere shortening is fastest during early life when rapid cell growth and division is needed for development, gradually slows down until around age 50, then remains relatively stable in later decades. However, children start with much longer telomeres than adults and have robust cellular repair systems, so despite the rapid shortening during growth, their telomeres remain long enough for healthy cell function and quick healing. The visible effects of aging occur when telomeres become critically short and cellular repair capacity diminishes over decades.
This non-linear pattern is important because it suggests telomeres aren't just passively wearing down but are actively regulated by your body based on different life stages and needs. During youth, rapid cell division for growth means faster telomere shortening. In middle age, the rate slows as growth needs decrease. In later life, the rate stabilizes, suggesting your body has mechanisms to preserve remaining telomere length when possible.
This pattern also explains why simply measuring telomere length isn't a perfect predictor of how someone will age. Telomere length is one important piece of the aging puzzle, but it works alongside many other biological processes rather than determining everything on its own.
Genetic Factors
Much of the variation in telomere length between individuals comes from genetic factors. Multiple genes influence both your starting telomere length at birth and how quickly they shorten throughout life.
Think of it this way: some people inherit naturally longer telomeres while others begin with shorter ones. These baseline differences tend to persist throughout your entire lifespan. This means someone born with longer telomeres will likely maintain that advantage even as both people age and their telomeres shorten over time.

While genetics sets the foundation, environmental factors significantly influence how telomeres change over time:
Chronic Stress: Prolonged psychological stress has been associated with accelerated telomere shortening. Studies of caregivers, individuals with post-traumatic stress, and those experiencing chronic work stress show measurably shorter telomeres compared to those who experience less stress.
Why would stress affect cellular aging? Chronic stress triggers sustained release of stress hormones like cortisol, which can increase inflammation and oxidative damage. Both processes appear to accelerate telomere shortening.
Physical Activity: Research suggests that people who engage in consistent physical activity tend to have longer telomeres, though the optimal type, intensity, and duration of exercise for telomere health is still being studied.
Sleep Quality: Poor sleep patterns and sleep disorders have been linked to shorter telomeres in several studies which suggests that quality rest plays a role in cellular maintenance.
Nutritional Factors: Diet quality shows associations with telomere length, with higher consumption of antioxidant-rich foods (like berries, leafy greens, and colorful vegetables) and omega-3 fatty acids (for example: fish, walnuts, flaxseeds) being linked to longer telomeres, while processed foods and excess sugar are associated with shorter telomeres.
Environmental Exposures: Factors such as air pollution, smoking, and exposure to toxic chemicals have been associated with accelerated telomere shortening.
Telomeres play a particularly important role in stem cell function. Stem cells are responsible for replacing damaged or aging cells throughout your body, from skin and blood cells to muscle and organ tissue. When stem cells have critically short telomeres, their ability to divide and generate new cells becomes compromised.
This connection helps explain why telomere shortening contributes to age-related decline in tissue repair and regeneration. As stem cell populations become less functional due to telomere attrition, the body's capacity for healing and maintenance gradually diminishes.
Most adult cells have very low levels of telomerase, the enzyme that can add DNA sequences back to telomeres. However, certain cell types, including stem cells and immune cells, maintain higher telomerase activity to support their ongoing division requirements.
The discovery of telomerase initially sparked excitement about potential anti-aging interventions. In theory, if you could activate telomerase in all your cells, you might be able to prevent telomere shortening and extend cellular lifespan.
However, the reality is more complex and potentially dangerous. Many cancer cells hijack telomerase to achieve unlimited replicative potential, essentially becoming immortal and able to divide endlessly. This is partly what makes cancer so dangerous and difficult to treat. Simply activating telomerase throughout the body could theoretically extend cellular lifespan but might also dramatically increase cancer risk by giving damaged cells the ability to divide indefinitely rather than dying off as they should.
While telomere length provides valuable insights into cellular aging, it represents just one component of the complex aging process. Current research suggests that telomere length works best as a health indicator when considered alongside other biomarkers such as inflammatory markers, metabolic health indicators (blood sugar control, insulin sensitivity, lipid profiles, etc.), and epigenetic age assessments. These different biomarkers are important because they each capture different aspects of how your body is aging, providing a more complete picture than any single measurement alone.
The field of telomere research continues to evolve rapidly, with ongoing studies exploring how different interventions might influence telomere dynamics and what role these cellular markers play in various health conditions.
Rather than viewing telomeres as the single key to aging, understanding their biology helps us appreciate the intricate connections between lifestyle choices, cellular health, the aging process, and overall longevity. The story of telomeres reminds us that aging isn't simply the passage of time but rather a complex biological process influenced by both our genes and our choices. By supporting the factors that promote healthy telomere maintenance, you're also supporting the broader biological systems that contribute to longevity and vitality throughout life!


More Info: At WellPro, we’re building an AI-native clinical platform purpose-built to scale the next era of care: personalized, preventative, and data-driven. Our thesis is simple but bold; the future of care delivery will be patient-centered, proactive, longitudinal, and closed-loop. That demands infrastructure that can ingest multi-modal health data, generate insight, drive action, and continuously optimize based on outcomes.
We believe Agentic AI is central to making this vision real. Not just chat interfaces or “co-pilots,” but deeply embedded, goal-driven AI agents that operate within the clinical system itself, helping surface insights at the point of care, automate routine tasks, and ensure closed-loop follow-through on interventions.